We have dedicated R&D facilities to meet the objectives of product innovation and technology development.
|
 |
We possess extensive manufacturing and R&D infrastructure for carrying out drug development on novel Biotech products, and to cater to the commercial production of our Biogeneric drugs. All our facilities, for Biogenerics and Biotech products, conform to cGMP guidelines for carrying out sterile fermentation operations. Our facilities are designed for the bacterial, mammalian and viral based production inherent in our product development and commercial production activities.
Some of the facilities we currently have include:
|
| Bio-reactor / Fermenter capacities |
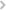 |
0.5 – 2 lit shakeflasks |
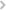 |
20 lit Fermenter |
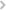 |
30 lit Disposable bioreactor |
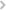 |
75 lit Fermenter |
|
| |
| Downstream facilities |
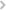 |
PCR |
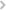 |
AKTA purifier |
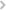 |
Micro-plate reader for carrying out regular ELISA, Fluorescent and Luminescent assays |
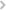 |
Ultra and High speed centrifuges
|
 |
HPLCs |
 |
Several chromatography apparatus |
 |
Inverted microscopes and spectrophotometers etc. |
|
| |
| We have a complete supporting infrastructure, including: |
 |
Lyophilizers |
 |
cGMP compliant pilot scale production facilities for recombinant microbial fermentation |
 |
Well organized cell banking system |
 |
Various cold storage facilities to store at - 80°, - 20°& 4° - 8° Celsius |
 |
Pure steam and pure water facilities |
 |
Standby power generator |
|
| |