| |
 |
| |
| |
|
 |
 |
Drug delivery systems have evolved over time and have become increasingly sophisticated yet more user-friendly, allowing drugs to be administered more reliably and easily. NDDS has seen a foray of transformations such as microencapsulation, epithelial and transdermal delivery, nanoparticles, liposomal vesicles etc.
Carrier-mediated drug delivery has emerged as a powerful methodology for the treatment of various pathologies. The therapeutic index of traditional and novel drugs is enhanced via the increase of specificity due to targeting of drugs to a particular tissue, cell or intracellular compartment, the control over release kinetics, the protection of the active agent or a combination of the above.
The global market for advanced drug delivery systems was more than €37.9 billion in 2000 and is estimated to grow and reach €75B by 2005 (i.e., controlled release €19.8B, needle-less injection €0.8B, injectable/implantable polymer systems €5.4B, transdermal €9.6B, trans-nasal €12.0B, pulmonary €17.0B, trans-mucosal €4.9B, rectal €0.9B, liposomal drug delivery €2.5B, cell/gene therapy €3.8B, miscellaneous €1.9B). Developments within this market are continuing at a rapid pace, especially in the area of alternatives to injected macromolecules, as drug formulations seek to cash in on the €6.2B worldwide market for genetically engineered protein and peptide drugs and other biological therapeutics. |
 |
| Oral Drug Delivery System |
| |
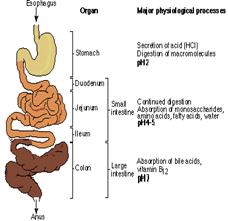
The choice of a delivery route is driven by patient acceptability, the properties of the drug (such as its solubility), access to a disease location, or effectiveness in dealing with the specific disease. The most important drug delivery route is the peroral route. An increasing number of drugs are protein and peptide based. They offer the greatest potential for more effective therapeutics, but they do not easily cross mucosal surfaces and biological membranes; they are easily denatured or degraded, prone to rapid clearance in the liver and other body tissues and require precise dosing. At present, protein drugs are usually administered by injection, but this route is less pleasant and also poses problems of oscillating blood drug concentrations. So, despite the barriers to successful drug delivery that exist in the gastrointestinal tract (i.e., acid-induced hydrolysis in the stomach, enzymatic degradation throughout the gastrointestinal tract by several proteolytic enzymes, bacterial fermentation in the colon), the peroral route is still the most intensively investigated as it offers advantages of convenience and cheapness of administration, and potential manufacturing cost savings. We are currently developing several proprietary Novel Drug Delivery Technologies for the oral delivery of peptides and proteins. Right now, we have separate delivery systems for Insulin, IgG and IgG-Fc fusion molecules, as well as a generic system for peptides and proteins.
TrabiORALTM
TrabiORALTM is an innovation focused on delivering proteins and peptides orally for a variety of human diseases. Transgene has developed a proprietary patented platform for delivering large and small proteins and peptides orally with demonstrated systemic efficacy.
 PROBLEMS WITH ORAL PROTEIN DELIVERY PROBLEMS WITH ORAL PROTEIN DELIVERY
Proteolysis
Poor transportation Across the GI barrier Rate & Dosage??
OUR SOLUTION: Game-Changing Technology. Although many different methods have been tried including the use of absorption agents, liposomes and Lysine residue switching, All have failed for reasons that can be explained. TrabiORAL’s™ use of the body’s own natural uptake mechanisms herald a new path in therapeutic delivery of proteins and peptides of different sizes from 5.8 kD to 150 kD with varying clinical applications. |
| |
|
|
|
|
|
|
|
 |
| © 2011. TRANSGENE BIOTEK LIMITED., All Rights Reserved. |
Designed by BitraNet |
|
|
|
 |
|