| |
 |
| |
| |
|
BREAST CANCER DRUG
 Disease Background Disease Background
Breast cancer is by far the most frequent cancer among women with an estimated 1.38 million new cancer cases diagnosed in 2008 (23% of all cancers), and ranks second overall (10.9% of all cancers). 1 in 10 of all new cancers diagnosed worldwide each year is breast cancer and is the most common cancer in women in both developing and developed areas. Incidence rates vary from 19.3 per 100,000 women in Eastern Africa to 89.7 per 100,000 women in Western Europe, and are high (greater than 80 per 100,000) in developed regions of the world (except Japan) and low (less than 40 per 100,000) in most of the developing regions.It is also the principle cause of death from cancer among women globally.
 Progress Progress
| » |
TBL-0905 is a novel molecule with shRNA delivered by a mutant and proprietary rAAV vector targeted at metastatic Breast cancer. |
| » |
TBL-0905 is our target gene, over-expressed in Breast Cancer, abnormally elevated in breast cancer cells and absent in normal cells. It is also known that lower levels of TBL-0905 relieve the epigenetic silencing of tumor suppressor genes. |
| » |
Transgene has cloned the required Adenoviral genes in a plasmid, and hence no adenoviral contaminations in the purified AAV. |
| » |
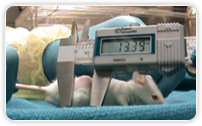
In the mouse xenograft studies, the drug demonstrated remarkable tumour regression (>75%) at the end of an 8 weeks study. |
|
LIVER CANCER DRUG (Hepatocellular Cancer)
 Disease Background Disease Background
Hepatocellular carcinoma (HCC) is a primary malignancy of the hepatocyte, generally leading to death within 6-20 months. Hepatocellular carcinoma frequently arises in the setting of cirrhosis, appearing 20-30 years following the initial insult to the liver. However, 25% of patients have no history or risk factors for the development of cirrhosis. The extent of hepatic dysfunction limits treatment options, and as many patients die of liver failure as from tumor progression.
Hepatocellular carcinoma is the fifth most common cancer in men and the eighth most common cancer in women worldwide. An estimated 560,000 new cases are diagnosed annually. The incidence of hepatocellular carcinoma worldwide varies according to the prevalence of hepatitis B and C infections. Areas such as Asia and sub-Saharan Africa with high rates of infectious hepatitis have incidences as high as 120 cases per 100,000.
Causes
Cirrhosis
In general, cirrhosis of any etiology is the major risk factor for hepatocellular carcinoma.
Alcohol
In the United States, about 30% of hepatocellular carcinoma cases are thought to be related to excessive alcohol use. Chronic alcohol use (>80 g/d or >6-7 drinks per day) for more than 10 years increases risk of hepatocellular carcinoma 5-fold.
Hepatitis B virus
Global incidence of chronic HBV infection is estimated to be 350 million persons; chronic HBV infection is the most common cause of hepatocellular carcinoma worldwide. Chronic infection in the setting of cirrhosis increases the risk of hepatocellular carcinoma 1000-fold.
Hepatitis C virus
It has become the most common cause of hepatocellular carcinoma in Japan and Europe, and it is also responsible for the recent increased incidence in the United States. The lifetime risk of hepatocellular carcinoma in patients with HCV is approximately 5%, appearing 30 years after infection.
 Scientific Rationale & Progress Scientific Rationale & Progress
TBL has developed a novel drug using our patented miRNA technology, delivered through our proprietary AAV vector system, which ‘silences’ the specific gene that causes the disease.
Due to its high specificity and novel delivery, the drug appears to have an exciting potential with high safety and efficacy resulting in the quality and longevity of this cancer sufferers.
The drug has demonstrated remarkable tumor regression in in-vitro and animal efficacy studies.
| |
|
| Control |
TBL-0905 |
 |
 |
With robust in-vivo data in hand, TBL is preparing to advance this drug to pre-clinical toxicology studies.
|
|
|
|
|
|
|
|
 |
| © 2011. TRANSGENE BIOTEK LIMITED., All Rights Reserved. |
Designed by BitraNet |
|
|
|
 |
|